The Obama administration awarded a coveted research grant to a financially strapped company working to put genetically modified (GM) salmon on American dinner tables, overlooking disclosures that the firm could run out of cash in early 2012, it has emerged.
Campaigners say the $500,000 grant to AquaBounty amounts to a bail-out for the firm's main investor, the business tycoon and former economics minister of Georgia, Kakha Bendukidze. They are also comparing it to the Solyndra controversy, which saw a solar company go bankrupt after receiving government loan guarantees.
"Certainly this does have shades of Solyndra. We have seen this company's stock plummeting for months and months – years actually – and what does the US Department of Agriculture (USDA) do but give this company money?", said Colin O'Neil, a policy analyst at the Centre for Food Safety, which opposes GM salmon.
"This is research that any public university or independent institution could be doing, so why is the USDA funding this interested company to do it?" he said.
The grant, awarded last month, comes at a critical juncture for AquaBounty.
After $67m and 16 years' waiting, the Food and Drug Administration could pronounce GM salmon fit for human consumption within weeks, the company's chief executive, Ronald Stotish, said.
"Based on what we are seeing we believe we will have an approval by end of this year but we plan for all contingencies," he said.
He said the company had prospective fish farmers lined up for the GM salmon in South Dakota, West Virginia, Wisconsin and Ohio. "We have people in the United States who are interested in growing these fish right now."
If approved, the salmon would be the first modified animal to make its way into the food chain, clearing the way for an entire menagerie of redesigns, from fast-growing trout and tilapia to the "enviro-pig", genetically altered to produce less polluting poo.
The USDA said it had followed the proper procedures in making the grant from the National Institute of Food and Agriculture (NIFA) – including a review of AquaBounty's financial information.
"On this particular grant, our procedures did call for the company to submit two years of financial information, including annual reports, tax forms, and other miscellaneous information. AquaBounty has provided this information for the grant they were awarded this year and are in compliance with all NIFA requirements for funding," the USDA spokeswoman wrote in an email.
She said NIFA reviewed 58 biotech research proposals before announcing the grants to AquaBounty and other companies.
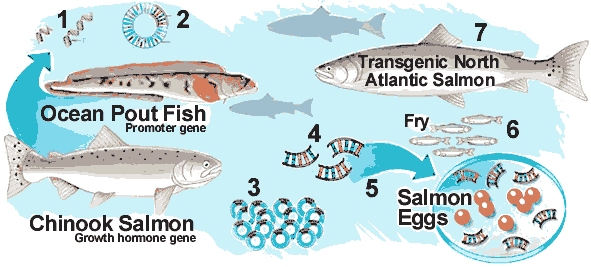
GM salmon, originally devised by researchers at Newfoundland's Memorial University, combine a growth hormone gene from the Chinook salmon, the largest variety in the Pacific, with a strip of DNA from the ocean pout, an eel-like animal that lives in extremely cold water.
Normally, the gene ensures the pout does not freeze to death. In the case of GM salmon, it ensures the growth hormone gene is switched on continuously for a non-stop growth spurt. The GM salmon grow up to six times as fast as the conventional variety.
The company plans to grow the modified salmon eggs at a lab in Prince Edward Island, and then fly them to Panama where they will be raised an inland fish farms. They would then be shipped back for sale in the US.
The use of inland fish farms is designed to prevent the salmon for escaping into the wild. The company says 98% of the fish are sterile.
However, the Canadian government has admitted it can not fully protect wild fish stocks in Canada from GM salmon, according to documents this week obtained by the Vancouver Sun. And in Alaska, senators introduced bills on Monday to ban the sale and shipment in the US of GM salmon, citing risks to salmon in the wild.
The grant to AquaBounty, though just a fraction of the $500m loan guarantee to the bankrupt solar company, comes at a time when the Obama administration is on the defensive when it comes to its handling of energy and environmental projects.
Emails released by the White House suggest that Obama fundraisers influenced the decision to fund Solyndra.
Another set of emails obtained by environmental organisations suggestthe State Department had an overly friendly relationship with lobbyists for the Keystone XL project, intended to pipe crude from the Alberta tar sands to Texas.
In the case of AquaBounty, campaigners argue there is a conflict of interest in funding research on GM animals by companies designing those animals.
As with other biotech companies, government grants have been crucial for AquaBounty's survival. Over the years, it has received some $3m from the US government and some $6m in funds from Canadian government.
"My sense is that they have been waiting years and years for something they could actually sell," said Patty Lovera, assistant director of Food and Water Watch which opposes GM salmon.
Stotish acknowledged the importance of government support. "It is true that we don't have unlimited funds," he said. "We are a small company so these grants are important to us."
The company's interim financial report, issued on 23 September, just five days before the grant announcement, records a net operating loss of $2.8m for the first six months of this year, $500,000 more than the previous year. "Current balances are sufficient to take the company into Q2 2012," the report says.
It adds: "The board is conscious however that the company's cash resources will need to be supplemented early in 2012."
The company's last round of fundraising in late 2010 saw Bendukidze take about 48% ownership with an investment of about $5m madethrough his investment firm Linnaeus Capital. The next largest owner is the Chilean investor, Alejandro Weinstein.
Stotish said the firm was looking to raise money again to take it beyond the first quarter of 2012. Even if the Food and Drug Administration (FDA) does sign off on AquaBounty, the company will still have to wait for approvals from the Canadian government to grow the fish eggs on a commercial basis, and from the Panamanian government.
There are no guarantees the FDA will approve GM salmon in the immediate future. A year ago, AquaBounty thought it was finally entering the end game after the FDA said the fish was safe for human consumption and did not pose a threat to the environment – but then the process unaccountably stalled.
"They are still not in the home stretch even if there is FDA approval," O'Neil said.
============================================
Aquabounty report http://www.aquabounty.com/documents/corporate/AquaBountyAdmission.pdf
================================================
17:42/03. OBAMA ADMINISTRATION BAILS OUT FRANKENFISH FIRM: The Obama Administration apparently awarded a research grant to the financially strapped company working to put genetically modified (GM) salmon on American dinner tables, overlooking disclosures that the firm could run out of cash in early 2012.
Campaigners say the $500,000 grant to AquaBounty company amounts to a bail-out for the firm's main investor, the business tycoon and former Economics Minister of Georgia, Kakha Bendukidze. It is being compared to the Solyndra controversy, which saw a solar company go bankrupt after receiving government loan guarantees. "Certainly this does have shades of Solyndra. We have seen this company's stock plummeting for months and months – years actually – and what does the U.S. Department of Agriculture (USDA) do but give this company money?" said Colin O'Neil, a policy analyst at the Centre for Food Safety, which opposes GM salmon. "This is research that any public university or independent institution could be doing, so why is the USDA funding this interested company to do it?" he said.
The grant, awarded last month, comes at a critical juncture for AquaBounty. After $67 million and 16 years' waiting, the Food and Drug Administration could pronounce GM salmon fit for human consumption within weeks, the company's Chief Executive, Ronald Stotish, said. "Based on what we are seeing we believe we will have an approval by end of this year but we plan for all contingencies," he said.
If approved, the AquaBounty genetically engineered salmon would be the first genetically modified animal to make its way into the U.S. food chain, potentially clearing the way for an entire menagerie of redesigns, from fast-growing trout and tilapia to the "enviro-pig.” The company plans to grow the modified salmon eggs at a lab in Prince Edward Island, and then fly them to Panama where they will be raised in inland fish farms. They would then be shipped back for sale in the U.S. However, the Canadian government has admitted it can not fully protect wild fish stocks in Canada from GM salmon, according to documents this week obtained by the Vancouver Sun. For more information check out the 18 October article from The Guardian: www.guardian.co.uk/environment/2011/oct/18/gm-salmon-aquabounty.
================================================
Aug 21, 2011
01 August 2011: 38 Agricultural organizations have signed a letter to Congressional Leaders urging them to allow the Food and Drug Administration (FDA) to complete its review of the world’s first genetically engineered fish for human consumption.
The move follows a recent amendment to the Agriculture Appropriations Bill (HR2112) that would stop the FDA from spending appropriated funds to finalize its review of the fish.
AquaBounty Technologies AquaAdvantage Atlantic salmon includes a gene from the faster growing Pacific Chinook salmon, which enables it to reach maturity twice as quickly as standard Atlantic salmon.
In the letter addressed to John Boehner, Nanci Pelosi, Harry Reid and Mitch Mc Connell, the authors call on Congress to reject the amendment, which was approved by voice on June 15th with only a handful of the House of Representatives in attendance.
Science-based review should not be subject to political intervention
Here it might be prudent to point out that 2 of the signatories are the American Meat Institute and the Biotechnology Industry Organization (BIO).
 The
letter states: “
Preventing
regulators from
completing their
assessments will
dent the credibility
of the FDA’s
science-based
approval process”.
The authors of this
not so subtle letter
go on to say: “ We
do not write to
support or oppose
this specific
application, but
rather to register
our concern with the
House’s action,
which, if allowed to
become law, would
disrupt the FDA’s
congressional
mandate to base its
assessment of human,
and animal drugs,
devices, vaccines
and process
applications on the
best available
science”. Here it
may be prudent to
let the reader know
that the rDNA used
to genetically
engineer the fish is
to be reviewed by
the FDA as a “New
Animal Drug” or
NAD! (More on
this later)
The
letter states: “
Preventing
regulators from
completing their
assessments will
dent the credibility
of the FDA’s
science-based
approval process”.
The authors of this
not so subtle letter
go on to say: “ We
do not write to
support or oppose
this specific
application, but
rather to register
our concern with the
House’s action,
which, if allowed to
become law, would
disrupt the FDA’s
congressional
mandate to base its
assessment of human,
and animal drugs,
devices, vaccines
and process
applications on the
best available
science”. Here it
may be prudent to
let the reader know
that the rDNA used
to genetically
engineer the fish is
to be reviewed by
the FDA as a “New
Animal Drug” or
NAD! (More on
this later)
The authors then go on to cite comments from Dr Calestous Juma of Harvard’s Kennedy School of Government, which were made at a recent hearing on agricultural biotechnology: “It is not this particular fish that is at stake. It is the principle behind the amendment and its wider ramifications. It sends the message to the rest of the world that that the science based regulatory oversight as embodied in the FDA review process is subject to political intervention”. (You don’t say! MOST definitely more on this later)
Politicians have to play the hand that they are dealt
 The
amendment to block
the approval process
was proposed by
House Republican Don
Young from Alaska
who had concerns
that the transgenic
salmon could
threaten wild salmon
populations, a
concern that has
been denied by
AquaBounty. Speaking
in the House on June
15th Young said, “It
is crucially
important we
understand that this
should not be
allowed, for the FDA
to say, okay, a
genetically raised
salmon – I call it a
Frankenstein fish, –
should never be
allowed in our
markets”. He was
backed by others who
claimed the fish
could have ”grave,
unintended
consequences on
human health.
Preliminary studies
show that the
compounds in
genetically
engineered salmon
may be linked to
cancer and severe
drug allergies“. And
yet another who said
“Because genetically
engineered salmon
are more sexually
aggressive and
resistant to
environmental
toxins, their escape
would pose a
catastrophic threat
to wild salmon
populations”.
(Clearly SOME in the
House take the time
to read their memo’s
after all!)
The
amendment to block
the approval process
was proposed by
House Republican Don
Young from Alaska
who had concerns
that the transgenic
salmon could
threaten wild salmon
populations, a
concern that has
been denied by
AquaBounty. Speaking
in the House on June
15th Young said, “It
is crucially
important we
understand that this
should not be
allowed, for the FDA
to say, okay, a
genetically raised
salmon – I call it a
Frankenstein fish, –
should never be
allowed in our
markets”. He was
backed by others who
claimed the fish
could have ”grave,
unintended
consequences on
human health.
Preliminary studies
show that the
compounds in
genetically
engineered salmon
may be linked to
cancer and severe
drug allergies“. And
yet another who said
“Because genetically
engineered salmon
are more sexually
aggressive and
resistant to
environmental
toxins, their escape
would pose a
catastrophic threat
to wild salmon
populations”.
(Clearly SOME in the
House take the time
to read their memo’s
after all!)
 Congressman
Jack Kingston was
the lone voice who
urged the House to
allow the FDA to
make a science-based
assessment, saying:
“We’re constantly
getting on the FDA
to use more sound
science, less
politics, have more
transparency, and it
appears that that’s
what they’re doing
here. And they may
come out against
genetically modified
salmon, but they are
just looking at it
right now to
determine”. (Clearly
this man hates
salmon)
Congressman
Jack Kingston was
the lone voice who
urged the House to
allow the FDA to
make a science-based
assessment, saying:
“We’re constantly
getting on the FDA
to use more sound
science, less
politics, have more
transparency, and it
appears that that’s
what they’re doing
here. And they may
come out against
genetically modified
salmon, but they are
just looking at it
right now to
determine”. (Clearly
this man hates
salmon)
Needless to say the House passed the amendment, (albeit on the technicality of not spending appropriated funds) effectively stopping the FDA from conducting the revue. So, without actually opening the Genetically Modified Can of “Worms” (to catch the fish with!) they took the easier way out, the temporary fix, but thank you, the fish was effectively contained. For then, for now. (more on this later)
Jim Greenwood, chief executive of BIO warned that interfering with the regulatory process in this way would also set a dangerous precedent. “ Disrupting FDA’s science-based assessment process based on non-scientific political concerns would set a dangerous precedent in our country. It is critical that the scientific experts who work within the FDA be allowed to conduct comprehensive scientific approvals without political influence”.
Ronald Stotish, CEO of AquaBounty is clearly frustrated by what he terms” The intervention of politicians in the regulatory process” and goes on to accuse the senators of trying to derail the approval of his transgenic fish and of “willfully ignoring science-based research and spreading misinformed paranoia”. He rails on: “The data is out there, although it has been ignored by all of the opposing groups – the FDA has already concluded that there is no food safety or environmental risk. But a science based review is being threatened by political shenanigans”. (My goodness man, hold yourself together! Just a moment, did you just say that the FDA has “already concluded”…? Did you just use the word “science”, again? More on this later folks)
AquaBounty claims that the transgenic salmon are sterile, exclusively female and unable to breed even if they do escape into the wild.
Subsequently though, an insider source has admitted that up to 5% are NOT sterile…….
AquaBounty CEO Stotish uses the words “science-based” and “already” an awful lot for someone who has in excess of $150 million riding on an as yet UN-reviewed ( according to both AquaBounty AND the FDA) assessment of this frankly fishy smelling New Animal Drug, don’t you think?
And lastly, (before I talk about the actual data on the fishy application) there is this gem from the FDA, based on it’s analysis of the AquaBounty technology published last September: “The food from AquaVantage Salmon that is the subject of this application is as safe as food from conventional Atlantic Salmon….In addition, no effects on stocks of wild salmon are expected“. (Now HOW would they know this? Isn‘t this whole whine about the issue exactly because the FDA hasn‘t the means to take a closer look at this “ transgenicfrankenfishsalmoneelthing” !?)
Gasp! Clearly the FDA sees absolutely no problems with this fish, this concept or the new animal drug itself…..without having formally seen the paperwork! Based upon the “technology“, the FDA has clearly made up it’s collective “Scientific Mind” about this in advance of the “scientific approval process ” so lamented as being “obstructed” by lone voice in the House Jack Kingston!
Now: This assessment of a genetically engineered salmon is the FIRST ever evaluation of a GE animal and will set the precedent for future approvals of GE animals. The FDA should be most especially cognizant of the scientific quality of the data and the rigor of the analysis needed to do a proper safety assessment of GE animals in this case. (Clearly they are not!)
 FDA
has set the bar very
low indeed
FDA
has set the bar very
low indeed
There is sloppy science, excruciatingly small sample sizes (only 6 fish per group for the allergenicity study), indeed the allergin test methodology was SO broad as to be statistically irrelevant, and so brief as to appear more of an afterthought than any part of an actual process, irrelevant or otherwise! Questionable practices of data manipulation (incomplete information/ conflicting information with regard to the IGF-1 data) and desperately inadequate analysis of conclusions of growth hormone levels in the flesh of the fish due to NO data at all on growth hormones due to the use of insensitive test methodology! And my personal favorite: the part of the study that dealt with phenotypic characterization data, along with all nutrient and food safety assessment data was based on the SIX fish at the PEI facility, NOT in the Panama facility, (as per the documentation) where they expect to raise these little genetic mutations. Indeed, even AquaBounty acknowledges in the report that the factors related to the two different locations on the “AquaVantage phenotype is unknown!” Therefore the FDA seems willing to conclude that there will be NO animal or human problems OR safety concerns with Aqua frankenfishsalmoneelthing raised in Panama, or in Canada where it is rumored they may set up shop too, down the road.
 *Since
this data was
released it has been
announced that
Canada will be the
egg producing
capitol of
transgenicfrankenfishsalmoneelthings,
with NO preemptive
oversight from the
FDA.
*Since
this data was
released it has been
announced that
Canada will be the
egg producing
capitol of
transgenicfrankenfishsalmoneelthings,
with NO preemptive
oversight from the
FDA.
And this assessment on safety, (or lack thereof) by the FDA is based on….(?) NO DATA at all !!!! Unacceptable. The FDA MUST demand data on GE salmon produced under the SAME husbandry and rearing conditions as the salmon they expect to present to the public for consumption.
In no way possible at all does this analysis conform to the FDA standard for assessment of a New Animal Drug (NAD).
The FDA requires NAD’s to be shown to be safe for animals, humans and the environment. This has NOT been shown for the GE salmon. What HAS been shown despite the woefully incomplete data has raised legitimate concerns about the potential for serious human health issues, namely the increased risk of allergenic potency and the continued exposures of the general population to an additional hormone mimicking byproduct of this transgenicfrankenfishsalmoneelthing.
The study fell flat in other key areas too: there was nothing under the “General Health Observations” since there was no knowledge of the criteria used and therefore no way to assess the (lack of) findings this was a Fail.
Under the heading “Direct and Indirect Hormone Factor” the study failed here too along with this nonsense gem that we the people have heard a tad too many times in the last 20 years or so (Vioxx anyone? rBGH anyone?)…..here it is then: “ No biologically relevant differences were detected in the levels of gene product/salmon growth hormone,”……but again: this is based on sloppy science and data deprived data…insufficient evidence (empty boxes on forms) where there should have been a notation saying” SEE ATTACHED FOR COMPREHENSIVE DATA”…at the very least. The hormone study (by the way) was statistically irrelevant because:
 1.
The fish were all
under 2 ounces, when
2 ounces is the
minimum needed to
test the hormone
levels
1.
The fish were all
under 2 ounces, when
2 ounces is the
minimum needed to
test the hormone
levels
2. The fish should have been tested at their market size, that is to say, at the size they would be when eaten!
Frankly, people just don’t EAT 2 ounce salmon!
FDA should have dismissed this study as irrelevant to the question of the direct food consumption risk, based on the guppy antics of the samples. This is the equivalent of testing a 6 year old girl for ovulation hormone activity as a marker against which to treat her mother….it is simply irrelevant and of no scientific value or consequence!
Further, the growth hormone is IGF and DOES pose a risk to humans, due in part to the raised levels of IGF and other growth hormones in ALL food producing animals. The interesting thing here is that the FDA itself (in this assessment) admits mush by noting, “ IGF levels are closely linked to growth hormones….may pose a hazard to humans…..has been considered a hazard for human consumption following increased growth hormone levels in in food producing animals (a reference to the issue of IGF-1 levels in milk from cows treated with a recombinant bovine growth hormone aka rbGH ) SO, fraudulent science meets….MORE fraudulent science!
 Something
else that we the
people should be
questioning on a
deeper level at this
time is this: If the
stated reason for
creating a
genetically
engineered salmon is
to speed up the
growing time of the
salmon…..and if the
gene splice is
occurring with the
Chinook BECAUSE of
the Chinooks rapid
growth cycle…….then
WHY is it STILL
necessary to add a
GROWTH HORMONE to
this creation?
Doesn’t the one
negate the necessity
of the other, or
vice versa….?
Something
else that we the
people should be
questioning on a
deeper level at this
time is this: If the
stated reason for
creating a
genetically
engineered salmon is
to speed up the
growing time of the
salmon…..and if the
gene splice is
occurring with the
Chinook BECAUSE of
the Chinooks rapid
growth cycle…….then
WHY is it STILL
necessary to add a
GROWTH HORMONE to
this creation?
Doesn’t the one
negate the necessity
of the other, or
vice versa….?
FDA should have dismissed this study the first chance they got (despite initial squeals from AquaBounty about “proprietary information and attempts to suppress the data)yes, can you believe that? And now CEO Stotish is criticizing the senators, NOT the FDA…….although officially of course FDA has not “had a chance to review the study” and AquaBounty has not had a chance to defend it’s “science-based” multi million dollar frankenfishsalmoneelthing. Clearly though an agreement has been reached, a compromise made, a deal struck. With or without spending from “appropriated funds” the FDA has made their feelings (and approval ) of AquaBountyfrankenfishsalmoneelthing quite clear, have they not?
Which brings me to something we should all be aware of: the primary reason why the FDA is proposing to approve the transgenic DNA for the salmon as a New Animal Drug, and not, let’s say as a “genetically engineered fish organism” is simple….
 Once
approved, a NAD will
have NO POST Market
Surveillance! As a
product NOT required
to be labeled (as a
GMO product) the
transgenicfrankenfishsalmoneelthing
will simply disperse
across America, into
supermarkets,
restaurants, fish
(soup) stocks, pet
food and (heaven
forbid!) into Fish
Oil supplements
(omegas). (That is,
until the proposed
FDA ban on
supplements takes
hold and grows to
include fish oils).
But that is a
different kettle of
fish entirely!
Once
approved, a NAD will
have NO POST Market
Surveillance! As a
product NOT required
to be labeled (as a
GMO product) the
transgenicfrankenfishsalmoneelthing
will simply disperse
across America, into
supermarkets,
restaurants, fish
(soup) stocks, pet
food and (heaven
forbid!) into Fish
Oil supplements
(omegas). (That is,
until the proposed
FDA ban on
supplements takes
hold and grows to
include fish oils).
But that is a
different kettle of
fish entirely!
AquaBounty/AquaVantage and 37 OTHER industry heavyweights want to accuse the congress/senate of holding the “scientists at FDA” back from doing their jobs of approving this? They want to accuse the senate/congress of holding Science hostage to politics? Over this irrelevant, fraudulent piece of quassi pseudo science?
 Given
that Monsanto
heavyweight and
legal representative
Michael Taylor has
revolved through the
doors of the FDA and
Monsanto so many
times that even HE
must have trouble
remembering which
building his current
office is situated
in (for now he is
the FDA “ Food Czar,
courtesy of our
current president,
in whose garden
grows the most
lavish un-permitted
“ Victory
garden”….would the
Green Police really
stop there? One has
to wonder, but I
digress…..)
Given
that Monsanto
heavyweight and
legal representative
Michael Taylor has
revolved through the
doors of the FDA and
Monsanto so many
times that even HE
must have trouble
remembering which
building his current
office is situated
in (for now he is
the FDA “ Food Czar,
courtesy of our
current president,
in whose garden
grows the most
lavish un-permitted
“ Victory
garden”….would the
Green Police really
stop there? One has
to wonder, but I
digress…..)
Yes, given that Taylor is the current food czar, and given his moribund appetite for creating the pro biotech regulations (when working as legal council for Monsanto) that the industry would lobby for, and his subsequent implementation of those laws (once niftily ensconced on his “FDA throne”) (during one of many office changes) there is no doubt that he is salivating at the chance to approve the transgenicfrankenfishsalmoneelthing so revered by the Biotechnology Industry Organization (BOI) that they had to write a letter to the House full of warnings and undercurrents that, in some measure could, and should, be construed as a threat.
According to public interest attorney Steven Druker, who has studied the FDA’s internal files, “During Mr. Taylor’s tenure as Deputy Commissioner, references to the unintended negative effects of bioengineering were progressively deleted from drafts of the policy statements (over the protests of agency scientists) and a final statement was issued claiming that (a) GM foods are no riskier than others and (b) that the agency had no information to the contrary.”
 When
the FDA announced
it’s original policy
on GMO, the public
was not aware of any
internal dissent.
The policy boldly
claimed that there
was no information
to indicate that GM
foods were different
or more risky than
natural varieties.
Since the public
generally trusted
the FDA, they
assumed that no such
risks existed. But,
nearly a decade
later, a lawsuit
would make public –
for the first time –
the agencies
internal documents –
and they told a
very, very different
story.
When
the FDA announced
it’s original policy
on GMO, the public
was not aware of any
internal dissent.
The policy boldly
claimed that there
was no information
to indicate that GM
foods were different
or more risky than
natural varieties.
Since the public
generally trusted
the FDA, they
assumed that no such
risks existed. But,
nearly a decade
later, a lawsuit
would make public –
for the first time –
the agencies
internal documents –
and they told a
very, very different
story.
A man from a biotech company told author Michael Pollan, (that the biotech company)” should not have to vouchsafe the safety of biotech food. Our interest is in selling as much of it as possible. Assuring its safety is the FDA’s job”.
That man was Phil Angell, Director of Corporate Communications for Monsanto.
AquaBounty/ AquVantage……Monsanto…….FDA…. All share one thing in common: Fraudulent Science.
© 2011 Dinah Everett Snyder
References
1. William Reed Business Media: Agro Groups: Derailing GM fish review would dent FDA’s credibility
2. Seeds of Deception: Jeffrey Smith
3. Union of Concerned Scientists: Admin Docket No.FDA-201034-N-0001
4. Science at Risk: Internal memos and documents of the FDA
5. Alliance for Bio Integrity: Steven Druker: Docket No 00N-1396
6. “ Pre Market Notice Concerning BioEngineered Foods” 66 Fed. Reg 4706 (January18, 2011)
About the author
Dinah Everett Snyder is currently writing a book about the FDA titled “CrimesAgainstHumanity: Is FDA the Rogue Militia for Big Medicine, Big Agriculture & Big Money….and WHY it matters!”
Connect with Dinah on Facebook!
------------------------------------------------------------------------------------
World Food Day: Monsanto, Frankenfish, Occupy Wall Street
Today is World Food Day.
Occupy Wall Street isn’t only about the banks. Since it also focuses on corporate greed, our entire food system is built on greed.
Corporation goliaths spend millions on lobbying lawmakers. “Money talks; nobody walks,” as the saying goes. One hand reaches out with the cash wanting to advance their adgenda. Another hand reaches back, with it’s own adgenda in mind, and takes the money. The corporation just got what it wanted: influence in perhaps the FDA, USDA, EPA or the justice system. Laws get changed or weakened, a product approved, a new appointee who is in the corporation’s pocket, or someone in a federal agency looking the other way, dismissing studies or making reports disappear. There were two winners in this – corporate America and the government. The losers far outnumber them; they are the millions of people in this country.
Time to SPEAK OUT AGAINST companies like MONSANTO. They are out to control the world’s food. With their GMO (genetically modified crops) they’ve damaged the ecosystem with overuse of Round-Up, caused the rise of ‘super weeds’ which are now resistant to Round Up. They have sued small farmers for ‘patent infringement’ because the GM has cross-pollinated with non GM and have endangered the organic crops and the organic industry.
Their push into Latin America will destroy the centuries-old and venerated maize crop. They are inundating Africa and the Middle East, selling poor farmers with their pitch that GMOs will help them feed their starving families. In reality it will tie them to Monsanto for seed and this dangerous chemical, glyphosate.
Round-up is being reformulated to be stronger and more deadly to both weeds and the eco-system. Rumor has it that maybe 2-4-D, Atrazine (also known as Agent Orange) could be added to strenghthen the mix. Perhaps the recipe will contain some older chemicals. Whatever Monsanto does, however, it will just start another round of chemical resistance.

GMO Salmon, by Aqua Bounty, also known as “Frankenfish” is up for approval by the FDA. It is designed to grow 2 or three times as big as the wild fish. This in itself is totally tampering with nature (like other GM products) but even more dangerous. Their Frankenfish will be the first living thing to be genetically modified and it frightens me. There is NO telling what would happen (and it’s likely to) if a GM salmon got into the wild population. Wild salmon would probably be decimated before long as these ‘creatures’ would have the ability to over populate them.
More and
more
research is
indicating
that GMO
foods are
unhealthy,
cause
numerous
conditions
and are
highly
detrimental
to health.
To learn
more about
GMOs, go to:
http://www.responsibletechnology.org/
=================================================
Soros is a funder of Earthjustice, that litigates for PCFFA and IFR and other KBRA NGO's.
================================================
Soros buys 897,813 Monsanto shares, 11/17/10 St. Louis Business Journal, "Billionaire investor George Soros’ hedge fund bought 897,813 shares of Monsanto — his second-largest holding on a dollar basis — during the third quarter, The Wall Street Journal reports. The position is now valued at $312.6 million."
http://www.aquabounty.com/documents/corporate/AquaBountyAdmission.pdf
==============================================
In accordance with Title 17 U.S.C. section 107, any copyrighted material herein is distributed without profit or payment to those who have expressed a prior interest in receiving this information for non-profit research and educational purposes only. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml





